Semester IV
Chemistry –IV/ Comp Sc –IV /Electronics-IV
Paper 13
CHPT-404: Chemistry of s & p block elements, States of Matter and Phase Equilibrium
THEORY Marks: 100
Section A: Inorganic Chemistry-2 (30 Lectures)
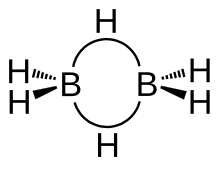
Unit I. General Principles of Metallurgy Chief modes of occurrence of metals based on standard electrode potentials. Ellingham diagrams for reduction of metal oxides using carbon as reducing agent. Hydrometallurgy. Methods of purification of metals (Al, Pb, Ti, Fe, Cu, Ni, Zn): electrolytic, oxidative refining, Kroll process, Parting process, van Arkel-de Boer process and Mond’s process. s- and p- Block Elements Periodicity in s- and p- block elements, w.r.t. electronic configuration, atomic and ionic size, ionization enthalpy, electronegativity (Pauling, Mullikan, and Alred-Rochow scales). Allotropy in C, S, and P. Oxidation states with reference to elements in unusual and rare oxidation states like carbides and nitrides), inert pair effect, diagonal relationship and anomalous behaviour of first member of each group. Compounds of s- and p- Block Elements Hydrides and their classification (ionic, covalent and interstitial), structure and properties with respect to stability of hydrides of p- block elements. Concept of multicentre bonding (diborane). Structure, bonding and their important properties like oxidation/reduction, acidic/basic nature of the following compounds and their applications in industrial, organic and environmental chemistry. Hydrides of nitrogen (NH3, N2H4, N3H, NH2OH) Oxoacids of P, S and Cl Halides and oxohalides: PCl3, PCl5, SOCl2 and SO2Cl2
Section B: Physical Chemistry-3 (30 Lectures)
Kinetic Theory of Gases Postulates of Kinetic Theory of Gases and derivation of the kinetic gas equation. Deviation of real gases from ideal behaviour, compressibility factor. Causes of deviation. van der Waals equation of state for real gases. Boyle temperature (derivation not required). Critical phenomena, critical constants and their calculation from van der Waals equation. Andrews isotherms of CO2.
Maxwell Boltzmann distribution laws of molecular velocities and molecular energies (graphic representation – derivation not required) and their importance. Temperature dependence of these distributions. Most probable, average and root mean square velocities (no derivation). Collision cross section, collision number, collision frequency, collision diameter and mean free path of molecules. Viscosity of gases and effect of temperature and pressure on coefficient of viscosity (qualitative treatment only).
Liquids Surface tension and its determination using stalagmometer. Viscosity of a liquid and determination of
coefficient of viscosity using Ostwald viscometer. Effect of temperature on surface tension and coefficient of viscosity of a liquid (qualitative treatment only)
Solids Forms of solids. Symmetry elements, unit cells, crystal systems, Bravais lattice types and identification of lattice planes. Laws of Crystallography - Law of constancy of interfacial angles, Law of rational indices. Miller indices. X–Ray diffraction by crystals, Bragg’s law. Structures of NaCl, KCl and CsCl (qualitative treatment only). Defects in crystals. Glasses and liquid crystals.
Chemical Kinetics The concept of reaction rates. Effect of temperature, pressure, catalyst and other factors on reaction rates. Order and molecularity of a reaction. Derivation of integrated rate equations for zero, first and second order reactions (both for equal and unequal concentrations of reactants). Half–life of a reaction. General methods for determination of order of a reaction. Concept of activation energy and its calculation from Arrhenius equation. Theories of Reaction Rates: Collision theory and Activated Complex theory of bimolecular reactions. Comparison of the two theories (qualitative treatment only).
Suggested Readings
Section A: 1. J. D. Lee : A new Concise Inorganic Chemistry, E L. B. S. 2. James E. Huheey, Ellen Keiter and Richard Keiter : Inorganic Chemistry: Principles of Structure and Reactivity, Pearson Publication.
Section B: 1 Barrow, G. M. Physical Chemistry Tata McGraw-Hill (2007). 2. Castellan, G. W. Physical Chemistry 4th Ed. Narosa (2004). 3. Mahan, B. H. University Chemistry 3rd Ed. Narosa (1998).
CHPP-404: Chemistry Laboratory Marks: 50
Section A: Inorganic Chemistry
Semi-micro qualitative analysis using H2S of mixtures not more than four ionic species (two anions and two cations and excluding insoluble salts) out of the following:
Cations : NH4+, Pb2+, Ag+, Bi3+, Cu2+, Cd2+, Sn2+, Fe3+, Al3+, Co2+, Cr3+, Ni2+, Mn2+, Zn2+, Ba2+, Sr2+, Ca2+, K+,
Anions : CO32– , S2 –, SO32 –, S2O32 –, NO3–, CH3COO–, Cl–, Br–, I–, NO3–, SO42-, PO43-, BO33-, C2O42-, F- (Spot tests should be carried out wherever feasible.)
Section B: Physical Chemistry
1. Surface tension measurement (use of organic solvents excluded)
a) Determination of the surface tension of a liquid or a dilute solution using a stalagmometer. b) Study of the variation of surface tension of a detergent solution with concentration.
2. Viscosity measurement (use of organic solvents excluded)
a) Determination of the relative and absolute viscosity of a liquid or dilute solution using an Ostwald’s viscometer. b) Study of the variation of viscosity of an aqueous solution with concentration of solute.
3. Phase equilibria
a) Construction of the phase diagram of a binary system (simple eutectic) using cooling curves. b) Determination of the critical solution temperature and composition of the phenol water system and study of the effect of impurities on it. c) Study of the variation of mutual solubility temperature with concentration for the phenol water system and determination of the critical solubility temperature.
Suggested Readings
1. Vogel’s Qualitative Inorganic Analysis, A.I. Vogel , Prentice Hall ,7th Edition. 2. Vogel’s Quantitative Chemical Analysis, A.I. Vogel , Prentice Hall ,6th Edition. 3. Senior Practical Physical Chemistry, B.D.Khosla, R. Chand & Co. Semester IV
Paper 14
PHPT – 404 Electricity, Magnetism and Electromagnetic Theory
THEORY Marks: 100
Electrostatics (Number of Lectures = 15)
Electric Field:- Concept of electric field lines and electric flux, Gauss’s law (Integral and differential forms), application to linear, plane and spherical charge distributions. Conservative nature of electric field E, irrotational field.
Electric Potential:- Concept of electric potential, relation between electric potential and electric field, potential energy of a system of charges. Energy density in an electric field. Calculation of potential from electric field for a spherical charge distribution.
Magnetostatics (Number of Lectures = 20)
Concept of magnetic field B and magnetic flux, Biot-Savart’s law, B due to a straight current carrying conductor. Force on a point charge in a magnetic field. Properties of B, curl and divergence of B, solenoidal field.
Integral form of Ampere’s law, applications of Ampere’s law: field due to straight, circular and solenoidal currents. Energy stored in magnetic field. Magnetic energy in terms of current and inductance. Magnetic force between two current carrying conductors. Magnetic field intensity.
Ballistic Galvanometer:- Torque on a current loop in a uniform magnetic field, working principle of B.G., current and charge sensitivity, electromagnetic damping, critical damping resistance.
Electromagnetic Induction and Electromagnetic waves (Number of Lectures = 25)
Faraday’s laws of induction (differential and integral form), Lenz’s law, self and mutual Induction.
Continuity equation, modification of Ampere’s law, displacement current, Maxwell’s equations in vacuum and dielectric medium, boundary conditions, plane wave equation: transverse nature of EM waves, velocity of light in vacuum and in medium, polarization, reflection and transmission.
Polarization of EM waves, Brewster’s angle, description of linear, circular and elliptical polarization.
Reference Books
1. Fundamentals of electricity and magnetism By Arthur F. Kip (McGraw-Hill, 1968) 2. Electricity and magnetism by J.H.Fewkes & John Yarwood. Vol. I (Oxford Univ. Press, 1991). 3. Introduction to Electrodynamics, 3rd edition, by David J. Griffiths, (Benjamin Cummings,1998). 4. Electricity and magnetism By Edward M. Purcell (McGraw-Hill Education, 1986) 5. Electricity and magnetism. By D C Tayal (Himalaya Publishing House,1988) 6. Electromagnetics by Joseph A.Edminister 2nd ed.(New Delhi: Tata Mc Graw Hill, 2006). 7.
PHPP-404: PHYSICS LABORATORY Marks: 50
1. To verify the Thevenin, Norton, Superposition, and maximum power transfer theorem. 2. To determine a low resistance by Carey Foster’s bridge. 3. To determine the (a) current sensitivity, (b) charge sensitivity, and (c) CDR of a B.G. 4. To determine high resistance by leakage method. 5. To determine the ratio of two capacitances by De Sauty’s bridge. 6. To determine self inductance of a coil by Anderson’s bridge using AC. 7. To determine self inductance of a coil by Rayleigh’s method. 8. To determine coefficient of Mutual inductance by absolute method.
Suggested Books for Reference
1.
B. L. Worsnop and H. T. Flint, Advanced Practical Physics, Asia Publishing House, New Delhi.
2.
Indu Prakash and Ramakrishna, A Text Book of Practical Physics, Kitab Mahal, New Delhi.
3.
Nelson and Jon Ogborn, Practical Physics.
Paper 15
MAPT-404: Differential Equations
THEORY Marks: 100
Ordinary Differential equations
First order exact differential equations. Integrating factors, rules to find and integrating factor. First order higher degree equations solvable for x,y,p=dy/dx. Methods for solving higher-order differential equations. Basic theory of linear differential equations, Wronskian, and its properties. Solving an differential equation by reducing its order. Linear homogenous equations with constant coefficients. Linear non-homogenous equations. The method of variation of parameters, The Cauchy-Euler equation. Simultaneous differential equations, total differential equations. Applications of differential equations: the vibrations of a mass on a spring, mixture problem, free damped motion, forced motion, resonance phenomena, electric circuit problem, mechanics of simultaneous differential equations.
Partial Differential Equations
Order and degree of partial differential equations. Concept of linear and non-linear partial differential equations. Formation of first order partial differential equations. Linear partial differential equation of first order, Lagrange’s method, Charpit’s method. Classification of second order partial differential equations into elliptic, parabolic and hyperbolic throughillustrations only. Applications to Traffic Flow.Using Computer aided software for example, Matlab/ Mathematica/ Maple/ MuPadcharacteristics, vibrating string, vibrating membrane, conduction of heat in solids, gravitational potential, conservation laws
Recommended Books
1. Shepley L. Ross: Differential equations, Third edition, John Wiley and Sons, 1984
2. I. Sneddon: Elements of partial differential equations, McGraw-Hill, International Edition, 1967.
Semester IV
Paper 16
LSPT 202 - BIOLOGY-2
THEORY Marks: 100
Cell and Cellular Processes
Unit 1. Techniques in Biology (Ch 1 Sheeler) (12 Periods)
Principles of microscopy; Light Microscope; Phase contrast microscopy; Fluorescence microscopy; Confocal microscopy; Sample Preparation for light microscopy; Electron microscopy (EM)- Scanning EM and Scanning Transmission EM (STEM); Sample Preparation for electron microscopy; X-ray diffraction analysis
Unit 2. Cell as a unit of Life (Ch 6 Campbell) (10 Periods)
The Cell Theory; Prokaryotic and eukaryotic cells; Cell size and shape; Eukaryotic Cell components
Unit 3. Cell Organelles (Ch 15, 16, 17,18,19,20 Sheeler) (22 Periods)
• Mitochondria: Structure, marker enzymes, composition; mitochondrial biogenesis; Semiautonomous organelle; Symbiont hypothesis; Proteins synthesized within mitochondria; mitochondrial DNA
• Chloroplast Structure, marker enzymes, composition; semiautonomous nature, chloroplast DNA
• ER, Golgi body & Lysosomes Structures and roles. Signal peptide hypothesis, N-linked glycosylation, Role of golgi in O-linked glycosylation. Cell secretion, Lysosome formation.
• Peroxisomes and Glyoxisomes: Structures, composition, functions in animals and plants and biogenesis
• Nucleus: Nuclear Envelope- structure of nuclear pore complex; chromatin; molecular organization, DNA packaging in eukaryotes, euchromatin and heterochromatin, nucleolus and ribosome structure (brief).
Unit 4. Cell Membrane and Cell Wall (Ch 7 Campbell / Ch 15 Sheeler / Ch 3 Raven) (8 Periods)
The functions of membranes; Models of membrane structure; The fluidity of membranes; Membrane proteins and their functions; Carbohydrates in the membrane; Faces of the membranes; Selective permeability of the membranes; Cell wall
Unit 5. Cell Cycle: Interphase, Mitosis and Meiosis (Ch 12, 13 Campbell) (8 Periods)
Role of Cell division; Overview of Cell cycle; Molecular controls; Meiosis
SUGGESTED BOOKS
1. Campbell, N.A. and Reece, J. B. (2008) Biology 8th edition, Pearson Benjamin Cummings, San Francisco. 2. Raven, P.H et al (2006) Biology 7th edition Tata McGrawHill Publications, New Delhi 3. Sheeler, P and Bianchi, D.E. (2006) Cell and Molecular Biology, 3rd edition, John Wiley & sons NY
LSPP 202 - BIOLOGY-2 Laboratory Marks: 50
1. To study prokaryotic cells (bacteria), viruses, eukaryotic cells with the help of light and electron micrographs. 2. Study of the photomicrographs of cell organelles 3. To study the structure of plant cell through temporary mounts. 4. To study the structure of animal cells by temporary mounts-squamous epithelial cell and nerve cell. 5. Preparation of temporary mounts of striated muscle fiber 6. To prepare temporary stained preparation of mitochondria from striated muscle cells /cheek epithelial cells using vital stain Janus green. 7. To prepare temporary stained squash from root tips of Allium cepa and to study the various stages of mitosis. 8. Study the effect of temperature, organic solvent on semi permeable membrane. 9. Demonstration of dialysis of starch and simple sugar. 10. Study of plasmolysis and deplasmolysis on Rhoeo leaf. 11. Measure the cell size (either length or breadth/diameter) by micrometry. 12. Study the structure of nuclear pore complex by photograph (from Gerald Karp)
Semester V
Chemistry –V/ Comp Sc –V /Electronics-V
Paper 17
CHPT-505: Chemistry of d-block elements, Quantum Chemistry and Spectroscopy
THEORY Marks: 100
Section A: Inorganic Chemistry-3 (30 Lectures)
Unit 1: Transition Elements (3d series) General group trends with special reference to electronic configuration, variable valency, colour, magnetic and catalytic properties, ability to form complexes and stability of various oxidation states (Latimer diagrams) for Mn, Fe and Cu. Lanthanides and actinides: Electronic configurations, Oxidation states, colour, magnetic properties, lanthanide contraction, separation of lanthanides (ion-exchange method only).
Unit 2:Coordination Chemistry Valency Bond Theory (VBT): Inner and outer orbital complexes of Cr, Fe, Co, Ni and Cu (coordination numbers 4 and 6). Structural and stereoisomerism in complexes with coordination numbers 4 and 6. Drawbacks of VBT. IUPAC system of Nomenclature.
Unit 3:Crystal Field Theory Crystal field effect, Octahedra symmetry. Crystal field stabilization energy (CFSE), Crystal field effects for weak and strong fields. Tetrahedral symmetry. Factors affecting the magnitude of ∆. Spectrochemical series. Comparison of CFSE for Oh and Td complexes, Tetragonal distortion of octahedral geometry. Jahn-Teller distortion. Square planar coordination.
Section B: Physical Chemistry-4 (30 Lectures)
Unit 4: Quantum Chemistry & Spectroscopy Spectroscopy and its importance in chemistry. Wave-particle duality. Link between spectroscopy and quantum chemistry. Electromagnetic radiation and its interaction with matter. Types of spectroscopy. Difference between atomic and molecular spectra. Born-Oppenheimer approximation: Separation of molecular energies into translational, rotational, vibrational and electronic components. Postulates of quantum mechanics, quantum mechanical operators. Free particle. Particle in a 1-D box (complete solution), quantization, normalization of wavefunctions, concept of zero-point energy. Rotational Motion: Schrödinger equation of a rigid rotator and brief discussion of its results (solution not required). Quantization of rotational energy levels. Microwave (pure rotational) spectra of diatomic molecules. Selection rules. Structural information derived from rotational spectroscopy. Vibrational Motion: Schrödinger equation of a linear harmonic oscillator and brief discussion of its results (solution not required). Quantization of vibrational energy levels. Selection rules, IR spectra of diatomic molecules. Structural information derived from vibrational spectra. Vibrations of polyatomic molecules. Group frequencies. Effect of hydrogen bonding (inter- and intramolecular) and substitution on vibrational frequencies. Electronic Spectroscopy: Electronic excited states. Free Electron model and its application to electronic spectra of polyenes. Colour and constitution, chromophores, auxochromes, bathochromic and hypsochromic shifts.
Unit 5: Photochemistry Laws of photochemistry. Lambert-Beer’s law. Fluorescence and phosphorescence. Quantum efficiency and reasons for high and low quantum yields. Primary and secondary processes in photochemical reactions. Photochemical and thermal reactions. Photoelectric cells.
Suggested Readings
Section A: 1. J. D. Lee : A new Concise Inorganic Chemistry, E L. B. S. 2. James E. Huheey, Ellen Keiter and Richard Keiter : Inorganic Chemistry: Principles of Structure and Reactivity, Pearson Publication.
Section B: 1 Barrow, G. M. Physical Chemistry Tata McGraw-Hill (2007). 2. Castellan, G. W. Physical Chemistry 4th Ed. Narosa (2004). 3. Mahan, B. H. University Chemistry 3rd Ed. Narosa (1998).
CHPP-505: Chemistry Laboratory Marks: 50
Section A: Inorganic Chemistry
1. Estimation of the amount of nickel present in a given solution as 2. Bis(dimethylglyoximato) nickel(II) or aluminium as oximate in a given solution gravimetrically. 3. Estimation of (i) Mg2+ or (ii) Zn2+ by complexometric titrations using EDTA. 4. Estimation of total hardness of a given sample of water by complexometric titration. 5. To draw calibration curve (absorbance at λmax vs. concentration) for various concentrations of a given coloured compound and estimate the concentration of the same in a given solution. 6. Determination of the composition of the Fe3+ - salicylic acid complex / Fe2+ - phenanthroline complex in solution by Job’s method. 7. Determination of concentration of Na+ and K+ using Flame Photometry.
Section B: Physical Chemistry
1. Potentiometric measurements
a. Strong acid with strong base b. Weak acid with strong base c. Mohr’s salt with potassium dichromate 2. Conductometric measurements.
a. Determination of the cell constant. b. Study of the variation of molar conductivity of a strong electrolyte (KCl) and of a weak electrolyte (acetic acid) with concentration. Conductometric titrations for the following systems
(i) strong acid - strong base (ii) weak acid - strong base
3. Kinetic studies
Study of the kinetics of the following reactions by integrated rate method:
a. Acid hydrolysis of methyl acetate with hydrochloric acid, volumetrically or conductometrically b. lodide-persulphate reaction.
Suggested Readings
1. Vogel’s Qualitative Inorganic Analysis, A.I. Vogel , Prentice Hall ,7th Edition. 2. Vogel’s Quantitative Chemical Analysis, A.I. Vogel , Prentice Hall ,6th Edition. 3. Senior Practical Physical Chemistry, B.D.Khosla, R. Chand & Co.
CSPT 505: Computer Networks
THEORY Marks: 100
Basic concepts : Components of data communication, standards and organizations
Network Categories : Area Networks (LAN, WAN and MAN), Network Relationships (Client-Server, Peerto-Peer), Network Topologies (Bus, Ring, Star, Mesh)
Layered Communication Connectivity : Fundamentals of Layered Connectivity, OSI and TCP/IP Models, comparison of models, Network Addressing – Physical and Logical Addresses
Physical Layer : Cabling, Network Interface Card, Transmission Media
Devices- Repeater, Hub, Bridge, Switch, Router, Gateway
Data Link Layer : Framing techniques; Error Control; Flow Control Protocols;
Shared media protocols - CSMA/CD and CSMA/CA.
Network Layer : Virtual Circuits and Datagram approach, IP addressing methods – Subnetting; Routing Algorithms (adaptive and non-adaptive)
Application Layer : Application layer protocols and services - DNS, HTTP, WWW
Network Security : Common Terms, Firewalls, Virtual Private Networks
BOOKS RECOMMENDED:
[1] A.S. Tanenbaum, Computer Networks, 4th Edition, Pearson Education.
REFERENCE BOOKS
1. B.A. Forouzan: Data Communication and Networking, 4th Edition, Tata McGraw Hill. 2. D.E. Comer, Internetworking with TCP/IP, Vol. I, Prentice Hall of India 3. W. Stalling, Data & Computer Communication, Maxwell Macmillan International Edition. 4. D. Bertsekas, R. Gallager, Data Networks, 2nd edition, Prentice Hall of India.
CSPP-505- Computer Networks Lab. Marks: 50
PRACTICALS BASED ON CSPT-505
ELPT – 505: Communication Electronics
THEORY Marks: 100
Introduction : Block diagram of an electronic communication system, modulation and demodulation, electromagnetic spectrum band designations and applications. Waveform spectra and effect of filtering on complex signals.
Analog Modulation: Amplitude Modulation: Frequency spectrum of AM waves, average power, average voltage, modulation index, AM-modulator circuits (collector modulation), AM-demodulator (diode detector), single side band generation and detection.
Angle Modulation: Frequency and phase modulation, frequency spectrum of FM waves, intersystem comparisons (FM and AM), FM generation and detection
Frequency division multiplexing (FDM).
Transmitters and Receivers: Communication channels for AM and FM broadcast, AM and FM transmitter, tuned RF receiver, Superheterodyne receiver.
Pulse Analog Modulation: Sampling Theorem and Nyquist Criterion. Pulse Modulation: pulse amplitude modulation (PAM), pulse width modulation (PWM) and pulse position modulation (PPM). Time division multiplexing (TDM).
Digital Communication: Block Diagram of a PCM system, Qualitative description of noise in PCM systems, concept of ASK, FSK, PSK.
Introduction to Modern Communication Systems: Satellite Communication, Fibre Optic System, Cellular Telephone System
Suggested Books:
1. Analog and Digital Communications, H. Hsu, Schaum’s Outline Series, Tata McGraw-Hill. 2. Electronic Communication, L. Temes and M. Schultz, Schaum’s Outline Series, Tata McGrawHill. 3. Analog and Digital Communication Systems, M.J. Roden, Prentice Hall of India. 4. Communication Systems: Analog and Digital, R.P. Singh and S.D Sapre, Tata McGraw-Hill. 5. Communication Electronics, Principles and Applications, L.E. Frenzel, Tata McGraw-Hill. 6. Electronic Communication Systems, G. Kennedy and B. Davis, Tata McGraw-Hill.
ELPP – 505 Electronics Laboratory Marks: 50
5.1 To study AM - Generator and Detector circuit
5.2 To study FM - Generator and Detector circuit
5.3 To study AM Transmitter and Receiver
5.4 To study FM Transmitter and Receiver
5.5 To study Time Division Multiplexing (TDM)
5.6 To study Pulse Amplitude Modulation (PAM)
5.7 To study Pulse Width Modulation (PWM)
5.8 To study ASK, PSK and FSK