CHEMISTRY ASSIGNMENT
FOR ROLL NO. 4980-4991
Diborane
Diborane is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless and highly unstable gas at room temperature with a repulsively sweet odor. Diborane mixes well with air, easily forming explosive mixtures. Diborane will ignite spontaneously in moist air at room temperature. Synonyms include boroethane, boron hydride, and diboron hexahydride.
Diborane is a key boron compound with a variety of applications. The compound is classified as "endothermic", meaning that its heat of formation, ΔH°f is positive (36 kJ/mol). Despite a high thermodynamic instability, diborane is surprisingly nonreactive for kinetic reasons, and it is known to take part in an extensive range of chemical transformations, many of them entailing loss of dihydrogen.
SYNTHESIS OF DIBORANE
Diborane is a key boron compound with a variety of applications. The compound is classified as "endothermic", meaning that its heat of formation, ΔH°f is positive (36 kJ/mol). Despite a high thermodynamic instability, diborane is surprisingly nonreactive for kinetic reasons, and it is known to take part in an extensive range of chemical transformations, many of them entailing loss of dihydrogen.
SYNTHESIS OF DIBORANE
Extensive studies of diborane have led to the development of multiple syntheses. Most preparations entail reactions of hydride donors with boron halides or alkoxides. The industrial synthesis of diborane involves the reduction of BF3 by sodium hydride, lithium hydride or lithium aluminium hydride:[8]
Structure and bonding
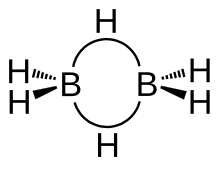
Diborane adopts a D2h structure containing four terminal and two bridging hydrogen atoms. The model determined by molecular orbital theory indicates that the bonds between boron and the terminal hydrogen atoms are conventional 2-center, 2-electron covalent bonds. The bonding between the boron atoms and the bridging hydrogen atoms is, however, different from that in molecules such as hydrocarbons. Having used two electrons in bonding to the terminal hydrogen atoms, each boron has one valence electron remaining for additional bonding. The bridging hydrogen atoms provide one electron each. Thus the B2H2 ring is held together by four electrons, an example of 3-center 2-electron bonding. This type of bond is sometimes called a 'banana bond'. The lengths of the B-Hbridge bonds and the B-Hterminal bonds are 1.33 and 1.19 Å respectively, and this difference in the lengths of these bonds reflects the difference in their strengths, the B-Hbridge bonds being relatively weaker. The weakness of the B-Hbridge vs B-Hterminal bonds is indicated by their vibrational signatures in the infrared spectrum, being ~2100 and 2500 cm−1, respectively. The structure is isoelectronic with C2H62+, which would arise from the diprotonation of the planar molecule ethene. Diborane is one of many compounds with such unusual bonding.
Of the other elements in Group IIIA, gallium is known to form a similar compound, digallane, Ga2H6. Aluminium forms a polymeric hydride, (AlH3)n, although unstable Al2H6 has been isolated in solid hydrogen and is isostructural with diborane.

VIKAS BHATI
NOTES ON GENERAL PRINCIPLES OF METALLURGY
- 8 BF3 + 6 LiH → B2H6 + 6 LiBF4
- 4 BCl3 + 3 LiAlH4 → 2 B2H6 + 3 LiAlCl4
- 4 BF3 + 3 NaBH4 → 2 B2H6 + 3 NaBF4
- 2 BH4− + 2 H+ → 2 H2 + B2H6
- 2 NaBH
4 + I
2 → 2 NaI + B
2H
6 + H
2
Structure and bonding

Diborane adopts a D2h structure containing four terminal and two bridging hydrogen atoms. The model determined by molecular orbital theory indicates that the bonds between boron and the terminal hydrogen atoms are conventional 2-center, 2-electron covalent bonds. The bonding between the boron atoms and the bridging hydrogen atoms is, however, different from that in molecules such as hydrocarbons. Having used two electrons in bonding to the terminal hydrogen atoms, each boron has one valence electron remaining for additional bonding. The bridging hydrogen atoms provide one electron each. Thus the B2H2 ring is held together by four electrons, an example of 3-center 2-electron bonding. This type of bond is sometimes called a 'banana bond'. The lengths of the B-Hbridge bonds and the B-Hterminal bonds are 1.33 and 1.19 Å respectively, and this difference in the lengths of these bonds reflects the difference in their strengths, the B-Hbridge bonds being relatively weaker. The weakness of the B-Hbridge vs B-Hterminal bonds is indicated by their vibrational signatures in the infrared spectrum, being ~2100 and 2500 cm−1, respectively. The structure is isoelectronic with C2H62+, which would arise from the diprotonation of the planar molecule ethene. Diborane is one of many compounds with such unusual bonding.
Of the other elements in Group IIIA, gallium is known to form a similar compound, digallane, Ga2H6. Aluminium forms a polymeric hydride, (AlH3)n, although unstable Al2H6 has been isolated in solid hydrogen and is isostructural with diborane.
VIKAS BHATI
NOTES ON GENERAL PRINCIPLES OF METALLURGY
No comments:
Post a Comment